Our Technology.,
|
Proprietary PlatformA powerful and versatile technology platform includes proprietary antibody-conjugated nanoparticles that are specifically adapted to antigen peptides that are only present in the bloodstream when the targeted disease is active.
|
Versatile and RapidDiagnostic tests take a matter of hours instead of days and weeks in some cases such as AFB culture tests. The platform can be adapted to different antigen targets and thus different diseases. Ultimately, we were be able to provide a rapid response to unknown diseases and outbreaks.
|
ProvenThe technology has been proven to work in thousands of patient samples for tuberculosis and HIV detection. It is shown to detect extremely low concentrations in blood and the ability to detect when other methods can not.
|
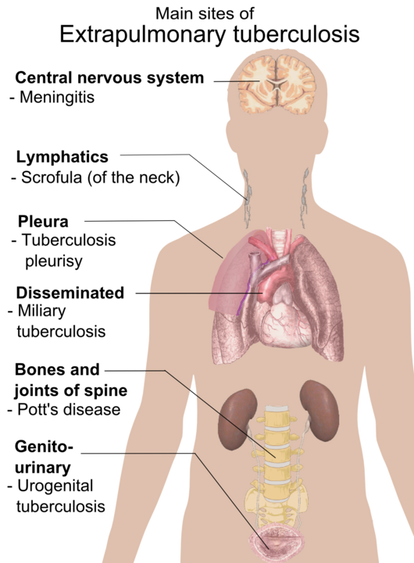
Tuberculosis Still a Global Health EpidemicTB remains a global epidemic with 10 million new cases and 1.5 million deaths annually. Better diagnostics are required to support global health efforts to effectively combat this disease. NanoPin's technology is uniquely qualified for active TB detection - we can detect both pulmonary and extrapulmonary TB in both adult patients and also much more difficult patient populations: children, infants, HIV co-infected.
|
HIV Millions Live with HIVNanoPin's technology has the potential to shorten detection windows and find active disease sooner and significantly improve patient treatment. HIV and TB are directly linked in that TB is the leading killer in HIV patients with 250,000 deaths annually (WHO, TB Annual Report). NanoPin's assays can ultimately be used for both HIV detection and the detection of TB in HIV patients.
|
Pulmonary and ExtraPulmonary Detection |
Learn More. How does the technology work?
Standard Approaches for Diagnosis of Infectious Disease and Their Problems
|
Sample collection and quality:
Standard disease diagnosis methods for infectious diseases require the isolation and analysis of pathogen-rich specimens, but this can be a problem when pathogens are present at low concentration despite high overall abundance or are not uniformly disturbed in diagnostic samples, or the site of infection is difficult to access or is unknown. |
Disease-specific protein biomarkers –sensitivity and specificity:
Pathogen-derived proteins present in the circulation, can serve as alternate means to diagnose infections, since some pathogens, including Mycobacterium tuberculosis (Mtb), shed proteins that can accumulate at low concentration in the circulation, regardless of the site of infection, as a result of normal tissue perfusion. Such proteins may be able to serve as disease-specific biomarkers, but they can be very difficult to detect, particularly early in infection, since they are usually present at low concentration and may be masked by interactions with abundant serum proteins, as well as specific antibodies generated in response to the infection. Closely related pathogens may also release highly conserved proteins that can be difficult to discriminate to allow species-specific identification. |
The NanoPin Solution
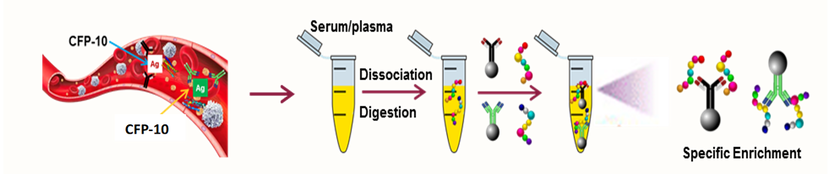
To address these issues, NanoPin uses an antibody enrichment approach to capture species-specific biomarker peptides released upon trypsin digestion of the serum of infected individuals and employs mass spectrometry to identify these peptides with high sensitivity and accuracy.
This approach has several advantages, since serum digestion cleaves all factors that may interact with a biomarker target to remove their masking effect and increase the amount of target protein available for analysis, while pathogen-derived peptides biomarker peptides produced during this digestion can be used to uniquely distinguish highly conserved proteins expressed by closely related pathogens. Finally, antibody-mediated enrichment of a target peptides prior to their analysis by tandem mass spectrometry serves to increase both the sensitivity and specificity of their detection.
This approach has several advantages, since serum digestion cleaves all factors that may interact with a biomarker target to remove their masking effect and increase the amount of target protein available for analysis, while pathogen-derived peptides biomarker peptides produced during this digestion can be used to uniquely distinguish highly conserved proteins expressed by closely related pathogens. Finally, antibody-mediated enrichment of a target peptides prior to their analysis by tandem mass spectrometry serves to increase both the sensitivity and specificity of their detection.
NanoPin’s TB Assay
TB was the leading cause of infectious disease mortality prior to the SARS-CoV-2 pandemic, which is expected disruption of global TB screening and treatment efforts caused by SARS-CoV-2 is expected to markedly worsen the number of new TB cases and deaths over the next five years.
Mycobacterium tuberculosis primarily causes respiratory infections that can progress to TB disease that remains contained within the lungs (pulmonary TB) or spread to other sites (extrapulmonary TB). Many individuals with pulmonary TB are not diagnosed by respiratory specimens (>40%) due to the low abundance of Mtb bacilli in these specimens (paucibacillary TB), while >15% of all TB disease is extrapulmonary TB, which must be diagnosed using invasive tissue biopsy by current methods. There is thus an urgent need for new assays that use non-respiratory samples such as serum to improve TB diagnosis.
Mycobacterium tuberculosis also belongs to a large family of mycobacteria (>180 species), and several of these nontuberculous mycobacteria (NTM) cause infections that resemble TB but require different drug interventions. These NTM species are often misdiagnosed as suspected TB cases and chronic, multi-drug resistant TB cases in some settings. Species-specific identification necessary to avoid ineffective treatment and drug resistance normally requires pathogen-rich samples.
However, peptides derived from the Mtb virulence factor CFP-10 permit species-specific identification, distinguish active TB disease from latent TB infections, and reflect viable pathogen load,1 unlike current methods that lack one or more of these abilities, including assays that detect Mtb-derived biomarker proteins.2
In NanoPin’s TB assay, serum samples from patients with suspected TB are trypsin digested and then spiked with a constant amount of a synthetic, isotope-labeled internal standard (IS) peptide of the same sequence as the CFP-10 target peptide (CPF10pep). These samples are then incubated with magnetic beads conjugated with a high-affinity monoclonal antibody that can capture and enrich both the CFP10pep and IS peptides.
Captured peptides eluted from the magnetic beads are then analyzed by high-resolution liquid chromatography coupled tandem mass spectrometry (LC-MS/MS) to identify and quantify the amount of Mtb-derived CFP-10 present in each patient’s serum sample.
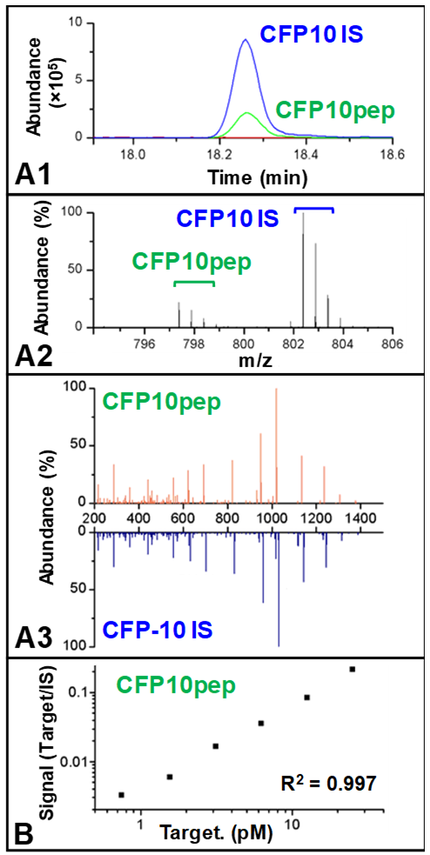
Since both CFP10pep and IS share the same sequence, both eluate as the same point in the LC gradient (Fig. A1), so that the high-intensity MS signal produced by the relatively high concentration spiked-in IS peptide standard can confirm the elution time of the CFP10pep, which may be present at very low concentration.
Mycobacterium tuberculosis-derived CFP10pep MS signal can be readily distinguished that of the IS peptide due to the greater mass of the latter peptide (Fig. A2), while CFP10pep-specific fragment ions detected in the MS/MS spectrum can be confirmed by comparison to the mass shifted peaks of the IS peptide (Fig. A3).
Finally, the amount of CFP-10 peptide present in a patients can be accurately quantified by comparing the ratio of the MS signal of CFP10pep and IS peptide to that of a standard curve generated when healthy human serum containing serial dilutions of recombinant CFP-10 are trypsin-digested, spiked with a constant amount of IS peptide, and analyzed by the same assay procedure (Fig. B). Standard curves generated in this manner reveal good linearity (R2 values >0.99) and allow the quantification of sub-picomolar concentration of CFP-10 to permit ultrasensitive detection of this TB biomarker in serum.
1 Liu, C. et al. Quantification of circulating Mycobacterium tuberculosis antigen peptides allows rapid diagnosis of active disease and treatment monitoring. Proc Natl Acad Sci U S A 114, 3969-3974, doi:10.1073/pnas.1621360114 (2017).
2 Flores, L. L. et al. Systematic review and meta-analysis of antigen detection tests for the diagnosis of tuberculosis. Clin Vaccine Immunol 18, 1616-1627, doi:10.1128/CVI.05205-11 (2011).
TB was the leading cause of infectious disease mortality prior to the SARS-CoV-2 pandemic, which is expected disruption of global TB screening and treatment efforts caused by SARS-CoV-2 is expected to markedly worsen the number of new TB cases and deaths over the next five years.
Mycobacterium tuberculosis primarily causes respiratory infections that can progress to TB disease that remains contained within the lungs (pulmonary TB) or spread to other sites (extrapulmonary TB). Many individuals with pulmonary TB are not diagnosed by respiratory specimens (>40%) due to the low abundance of Mtb bacilli in these specimens (paucibacillary TB), while >15% of all TB disease is extrapulmonary TB, which must be diagnosed using invasive tissue biopsy by current methods. There is thus an urgent need for new assays that use non-respiratory samples such as serum to improve TB diagnosis.
Mycobacterium tuberculosis also belongs to a large family of mycobacteria (>180 species), and several of these nontuberculous mycobacteria (NTM) cause infections that resemble TB but require different drug interventions. These NTM species are often misdiagnosed as suspected TB cases and chronic, multi-drug resistant TB cases in some settings. Species-specific identification necessary to avoid ineffective treatment and drug resistance normally requires pathogen-rich samples.
However, peptides derived from the Mtb virulence factor CFP-10 permit species-specific identification, distinguish active TB disease from latent TB infections, and reflect viable pathogen load,1 unlike current methods that lack one or more of these abilities, including assays that detect Mtb-derived biomarker proteins.2
In NanoPin’s TB assay, serum samples from patients with suspected TB are trypsin digested and then spiked with a constant amount of a synthetic, isotope-labeled internal standard (IS) peptide of the same sequence as the CFP-10 target peptide (CPF10pep). These samples are then incubated with magnetic beads conjugated with a high-affinity monoclonal antibody that can capture and enrich both the CFP10pep and IS peptides.
Captured peptides eluted from the magnetic beads are then analyzed by high-resolution liquid chromatography coupled tandem mass spectrometry (LC-MS/MS) to identify and quantify the amount of Mtb-derived CFP-10 present in each patient’s serum sample.
Since both CFP10pep and IS share the same sequence, both eluate as the same point in the LC gradient (Fig. A1), so that the high-intensity MS signal produced by the relatively high concentration spiked-in IS peptide standard can confirm the elution time of the CFP10pep, which may be present at very low concentration.
Mycobacterium tuberculosis-derived CFP10pep MS signal can be readily distinguished that of the IS peptide due to the greater mass of the latter peptide (Fig. A2), while CFP10pep-specific fragment ions detected in the MS/MS spectrum can be confirmed by comparison to the mass shifted peaks of the IS peptide (Fig. A3).
Finally, the amount of CFP-10 peptide present in a patients can be accurately quantified by comparing the ratio of the MS signal of CFP10pep and IS peptide to that of a standard curve generated when healthy human serum containing serial dilutions of recombinant CFP-10 are trypsin-digested, spiked with a constant amount of IS peptide, and analyzed by the same assay procedure (Fig. B). Standard curves generated in this manner reveal good linearity (R2 values >0.99) and allow the quantification of sub-picomolar concentration of CFP-10 to permit ultrasensitive detection of this TB biomarker in serum.
1 Liu, C. et al. Quantification of circulating Mycobacterium tuberculosis antigen peptides allows rapid diagnosis of active disease and treatment monitoring. Proc Natl Acad Sci U S A 114, 3969-3974, doi:10.1073/pnas.1621360114 (2017).
2 Flores, L. L. et al. Systematic review and meta-analysis of antigen detection tests for the diagnosis of tuberculosis. Clin Vaccine Immunol 18, 1616-1627, doi:10.1128/CVI.05205-11 (2011).